Genes and Aging


The Sept. 2006 issue of Nature Genetics has an interesting article by Siegfried Hekimi entitled "How Genetic Analysis Tests Theories of Animal Aging" (subscription needed). The phenomenon of aging raises a number of interesting ethical and social issues.
Recall that in an earlier post I noted that I have been giving some thought to the proposal that we ought to wage a war on aging itself. As I read through Hekimi's paper these issues came to me again. As a prioritarian I am of mixed minds about the prospect of such a war being waged, given the facts of scarcity and pervasive disadvantage. Much of course depends on what the proposed means of fighting such a war are, how proportionate our attention to this cause is relative to other pressing moral demands, as well as the likely magnitude of the benefits of waging such a war. I think such concerns inform, for example, the Longevity Dividend Campaign, hence the reason I support it.
The more I think about the phenomenon of aging the more I realise that we need to critically assess our current attitudes towards aging and the effort to extend the human health span. Here are a few extracts from Hekimi's paper that got me thinking about these kinds of concerns:
Aging. Aging is the increase in the probability of dying with the passage of time. It is also the increased susceptibility for any of a number of diseases, regardless of whether they are ultimately responsible for the deaths of the individuals developing them. In addition, aged individuals are less resistant to injury, whether from physiological accidents (for example, surviving a heart attack) or environmental accidents (for example, bone facture), and they are less resistant to infection. Aging is a phenotype (of which life span is one feature); consequently, the pattern of aging depends on the genotype and on the environment: different species, as well as different strains from a single species, such as the mouse, and different human populations, develop different physiological or anatomical alterations with age and die from different age-dependent diseases.
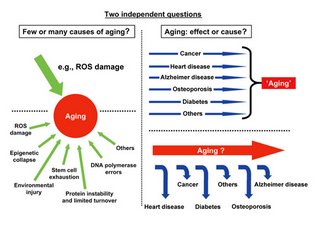
....Is what we call 'aging' a mechanism or concept? One can describe at least two views of the meaning of the term 'the aging process'(Fig. 2- [inserted below]). In one view, aging is a biochemical mechanism that does not induce disease in itself, but whose progression is an ever-increasing risk factor for both the onset and the severity of age-dependent diseases and leads to a lesser ability to resist, survive and recover from physiological disturbances. In the other view, the aging process is but the conceptualization of the collection of all age-dependent diseases. In a given species, such as humans, age-dependent diseases, whether immediately life-threatening or less severe at first, arise in a pattern that is relatively stereotyped within a population and is easily recognized as the consequence of increasing age, both by visible signs (graying, wrinkles, increased body fat, changes in posture) and by patterns of disease prevalence (such as atherosclerosis, cancer and, later in life, neurodegenerative diseases). Interestingly, the view that the molecular mechanism that underlies aging is the accumulation of unrepaired damage seems to be consistent with both views of aging. Indeed, in the first view, the effect of the accumulated damage is diffuse: it creates a decrease in the strength of homeostasis that leads to an increase in the risk of developing disease, but it does not initiate disease. In the other view, the damage directly produces disease, with different types of damage producing different diseases.
[Nature Genetics - 38, 985 - 991 (2006)]
Siegfried Hekimi
....Although the existence of mutants with increased life span could have been predicted based on evolutionary theory, the finding that there are mutations that can increase life span as massively as some do could not have been predicted. For example, mutations in the daf-2 or isp-1 genes of Caenorhabditis elegans can increase life span twofold, and this can be further doubled in combination with other single-gene mutations such as in daf-2 clk-1 double mutants4. As already discussed, it is invariably observed that toward the end of their life span, animals develop a variety of age-dependent diseases. In a given species, strain or population, a single disease can be a major cause of death, but avoiding that particular disease does not ensure indefinite survival, because some other lethal disease of later onset will have the opportunity to develop. This is illustrated by the finding that mice that are partially resistant to cancer do not really live longer and by the statistical prediction that if we could cure cancer or atherosclerosis in humans, life span would be lengthened by only a few years56. What to think, then, of a mutation in a single gene or pair of genes that can increase animal life span several-fold? Considering worm mutants that live several times longer than the wild type, one realizes that to obtain such an effect, every single degenerative process that normally kills wild-type worms must be dramatically delayed, and all the mechanisms of somatic maintenance and structural elements that have evolved to suffice for only one lifetime become capable of functioning and supporting life during several lifetimes. This observation implies that there are only a handful of processes that together are causal to all manifestations of aging, which is why mutations that can affect one or a few of these processes can have such large effects. Although the magnitude of the life span effects that have been obtained by genetic manipulation in mammals have not been as impressive as in invertebrates and caution is still required for the interpretation of some of the currently available data studies, the increases are sufficiently large (up to 50%) that it is arguable that this could not have been achieved if only a single disease process had been affected by these genetic changes.
Cheers,
Colin


<< Home